Agenda:
Warmup
Isotopes Phet
Stability Chart
Exit Slip
Warmup
Work with your team on whiteboards.
Study the following models of alpha decay. For each example, write down the number of protons and neutrons for each atom before and after the change. What is the pattern in how the nucleus changes for alpha decay?
Study the following models of beta decay. For each example, write down the number of protons and neutrons for each atom before and after the change. What is the pattern in how the nucleus changes for beta decay?
Isotopes Phet
Work with your team on whiteboards.
Study the following models of alpha decay. For each example, write down the number of protons and neutrons for each atom before and after the change. What is the pattern in how the nucleus changes for alpha decay?
Study the following models of beta decay. For each example, write down the number of protons and neutrons for each atom before and after the change. What is the pattern in how the nucleus changes for beta decay?
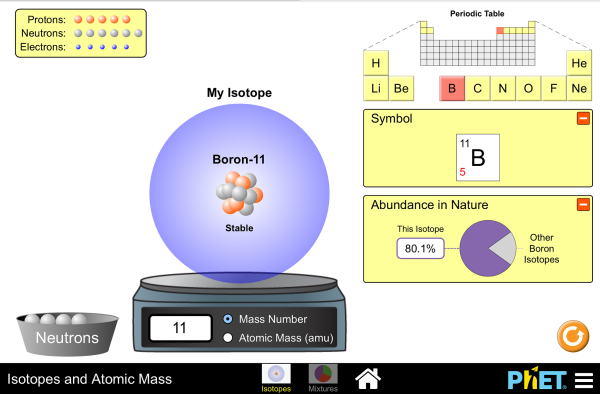
Isotopes Phet
Using the Phet simulation, try to answer the question: What makes an atom unstable?
Stability Chart
In your science journal, create a new entry entitled "Stability Chart." Copy and paste the chart below into your journal and use it to answer the questions.

Stability Chart
In your science journal, create a new entry entitled "Stability Chart." Copy and paste the chart below into your journal and use it to answer the questions.
A: Write down 3 observations describing the chart.
B: What does this graph tell us about the stability of atoms?
C: Use the graph to predict if the following atoms are stable. State WHY you think they are unstable based on the graph.- An atom with 20 protons and 20 neutrons.
- An atom with 100 protons and 100 neutrons.
- An atom with 80 protons and 120 neutrons.
- An atom with 60 protons and 40 neutrons.
- An atom with 40 protons and 80 neutrons.
D: Propose a stable atom. Write the following information about the proposed atom:
- Element Name
- # of Protons
- # of Neutrons
At the top of your journal entry, write a CER paragraph to answer the following question:
Why do you think atoms are radioactive?

No comments:
Post a Comment
Comments: